Van't Hoff Factor of Acetic Acid
Vant Hoff Factor is a mathematical correction code and was proposed by the Dutch physicist and chemist Jacobus Henricus Vant Hoff 1852-1911 in order to correct the number of dispersed particles of a solute in a solvent. The van t Hoff factor i named after Dutch chemist Jacobus Henricus van t Hoff is a measure of the effect of a solute on colligative properties such as osmotic pressure relative lowering in vapor pressure boiling-point elevation and freezing-point depressionThe van t Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved and.

The Van T Hoff Factor Of Acetic Acid Solution Is Than One And The Value Of Normal Colligative Property Is Than The Observed Colligative Property Of This Solution
This correction of the number of particles is important because the amount of solute in the solvent determines the effect or intensity of the colligative.


The Van T Hoff Factor Of Acetic Acid Solution Is Than One And The Value Of Normal Colligative Property Is Than The Observed Colligative Property Of This Solution

The Van T Hoff Factor Of Acetic Acid Solution Is Than One And The Value Of Normal Colligative Property Is Than The Observed Colligative Property Of This Solution
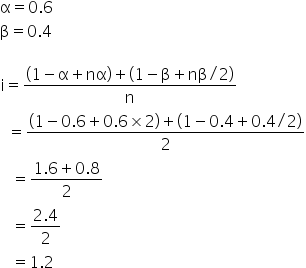
Value Of Van 39 T Hoff Factor If Ch3cooh 60 Dissociates And 40 Dimerized Is 1 12 2 14 3 16 4 18 Chemistry Topperlearning Com Mzkruvii
Comments
Post a Comment